Beer lambert law extinction coefficient example
𝜀 260 = extinction coefficient of the oligonucleotide at 260 nm in L ⋅ mol-1 Key to the Beer-Lambert law is the extinction coefficient, For example, for
Use Beer-Lambert Law to calculate the molar absorptivity of a single absorbing species. The equation is A = ecl, so the equation for molar absorptivity is e = A ÷ cl.
According to the Beer Lambert Law the ‘Absorbance’ is The proportionality constant of the equation is termed as the molar extinction coefficient of the
This page takes a brief look at how UV-visible absorption spectra can be used to help You also need to be familiar with the Beer-Lambert Law. For example, on
A beginners guide to laboratory spectrophotometry and the Beer-Lambert Law. Deriving the extinction coefficient from Example 1: Absorbance 1, extinction
K. we obtain a more familiar expression of the Beer-Lambert law.I k called an extinction coefficient. We often express Beer-Lambert mm^3 for example.
The Beer-Lambert law relates the attenuation of light to the properties of What is the extinction coefficient? SOLUTION. Using Beer-Lambert For example
Q: Cytosine has a molar extinction coefficient of 6*10 3 at 270 nm at pH 7. Calculate the absorbance and percent transmission of 1*10-4 and 1*10-3 M cytosine solution
Ocean Optics GmbH Sales, Beer’s Law. Beer-Lambert Law, ε λ = molar absorptivity or extinction coefficient of the chromophore at wavelength λ
A classic example of this phenomenon Extinction Coefficient, , having units of M-1cm-1, Spectrophotometry and the Beer-Lambert Law
… Beer’s Law and Molar Extinction Coefficients or biological molecule in a solution using the Beer-Lambert Law An example of a Beer’s Law
Beer’s Law Example. (Absorbance = e L c) when you were given the molar absorptivity constant (or molar extinction coefficient). In this equation,
… s Law or the Beer-Lambert Law and learn the equation Beer’s Law Definition and Equation Beer’s Law or (formerly called the extinction coefficient)
Calculating Nucleic Acid or Protein concentration using the Beer-Lambert law which relates Lambert equation, the extinction coefficients can be converted
The Beer–Lambert law, by definition of attenuation cross section and molar attenuation coefficient. Then the Beer–Lambert law becomes = An example is the
… Beer-Lambert Law and the Temperature Dependence of the Crystal Violet-Sodium Hydroxide Reaction. Beer-Lambert law, if the molar extinction Example CV
Calculating Nucleic Acid or Protein Concentration Promega

Oligonucleotide Quantification Sigma-Aldrich
Interpreting Nanodrop (Spectrophotometric) Results Where A=absorbance, ԑ=extinction coefficient, c=concentration and l=path length. The Beer‐Lambert law
The Beer-Lambert law for the absorption, A, Some DNA extinction coefficients are given in Table For example, the A 280 (1 mg/cm 3
Another name for molar absorptivity is the molar extinction coefficient. You would calculate the using the Beer-Lambert Law equation: A = ε . c . l Where: A
Absorption and Transmission of light and the Beer-Lambert Law Lecture 21 Absorbance and the extinction coefficient
… also known as Beer’s law, the Lambert–Beer a molar absorptivity (extinction coefficient) essay sample on The Beer-Lambert Law and Its Limitation
The equation for Beer’s law (εm) = slope or the molar extinction coefficient in beers law which has units How do you calculate concentration from absorbance?
For example, measuring the How are extinction coefficients determined for Proteins? The equation for the Beer-Lambert law can also be written as [ A
According to the Beer-Lambert law, the absorbance at a given wavelength of light (A) is proportional to the molar extinction coefficient (E), the concentration of

UV Vis Absorption Experiment 1: Beer- is known as the molar extinction coefficient and is a measure of the The Beer Lambert law is expressed formally
Beer–Lambert law has been listed as a in the article Extinction coefficient indicates that k has by using appropriate constants. for example,
Vertical leaf area distribution, light transmittance, and application of the Beer-Lambert Law in four mature canopy extinction coefficients (k),
Determine concentration Using Beer’s law. I don’t know how to determine the extinction coefficient and the concentration from the From the Beer-Lambert law
Beer’s Law and Molar Extinction Coefficient Background and Objectives Colorimeters Beer-Lambert Law: An example of a Beer’s Law plot Beers law Beer’s law
beers_law – Download as PDF Beer’s Law and Molar Extinction Coefficient. Beer-Lambert Law: An example of a Beer’s Law plot (concentration versus absorbance)
Lambert’s Law Example Beer-Lambert Law Lambert‟s and Beer‟s Laws are combined to describe The molar extinction coefficient (ε)

1 Lecture 2. The Beer-Bouguer-Lambert law. Concepts of extinction (scattering plus absorption) and emission. Schwarzschild’s equation. Objectives:
This formula is known as the Beer-Lambert Law, and the constant ε is called molar absorptivity or molar extinction coefficient and is a measure of the probability of
This is not an example of the work written by our professional academic writers. Beer-Lambert Law, λ is the molar absorbtivity coefficient in L mol-1 cm-1.
I already calculated protein concentration using Bradford estimation but I need to calculate molar extinction coefficient by beer Lambert law. will any one help me?
Gas Absorption: Beer-Lambert Law I ε≡extinction coefficient [L mol-1 cm-1] Example: UV Attenuation by O3 and O2
A beginners guide to laboratory spectrophotometry and the Beer-Lambert Law.
Spectrophotometry and the Beer-Lambert Law: A classic example What is the value of the molar extinction coefficient or molar absorptivity,
If the extinction coefficient is not known, Experiment C-28 Beer-Lambert law Ver 3.0.5 For example, the absorbance of a solution with an unknown
UV Vis Absorption Experiment 1 Beer- Lambert Law and
3 Experiment C-28 Beer-Lambert law Ver 3.0.8 coefficient ԑ, and the path length l into the equation. If the extinction coefficient is not known, a calibration curve
Calculating concentration using Beer Lambert Law? I am trying to calculate the concentration of a solution using the Beer Lambert Law. extinction coefficient and
An explanation of the Beer-Lambert Law, and the terms absorbance and molar absorptivity (molar absorption coefficient)
Beer Lambert Law solved problems Given the absorbance coefficient of trp is 6.4 × 10 3 Extinction coefficient of NADH at 340 nm is 6440 L/mol/cm. whereas NAD
Fluorescence Fundamentals is defined by the Beer-Lambert law A=EC on the environment than absorption spectra and extinction coefficients. For example,
Integrating by separation of variables we obtain the Beer-Lambert extinction coefficient (m2 kg-1). Now we Beer-Lambert Law only holds when we perfectly
Absorbance Spectroscopy and Beer’s Law known as the extinction coefficient , The Beer-Lambert Law is the basis of using absorbance measurements to determine
Here is an online Beer Lambert Law calculator for you to calculate the absorbance using Beer’s Law with ease. Just enter the values of molar absorption coefficient
If we have for example I recommend to reformulate Beer-Lambert’s law in a way across the sample investigated, and the molecular extinction coefficient
How can I calculate the absorption coefficient from an absorbance you shall be able to determine extinction coefficient per mole the Beer-Lambert law, – effective public relations and media strategy pdf A combination of the above two laws i.e. the Lambert law and Beer’s law yields the (or molar extinction coefficient). again if you can show me an example
15/10/2010 · Spectrophotometry, transmittance, absorbance and the Beer-Lambert Law. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science
Interactive Video Case Study: Beer-Lambert law – Linear calibration. Produced by Graham Currell, University of the West of England, Bristol in association with:
Design Project on Beer- Lambert’s Law. This is not an example of the work written (where L is the length of medium and k1 is molar extinction/absorption co
Molar extinction coefficient e has units of M-1 cm-1 and is a constant of proportionality and A are derived from the Beer-Lambert law For example, if a sample
12/01/2013 · – Beer’s Law as described by my former Beer’s Law and the Beer-Lambert equation in I wikipedia’d absorbance and extinction coefficient,
Answer the following questions: Question 1 Use the experimental data to calculate a ‘best-estimate’ for the concentration, C o, of the test solution.
The Beer-Lambert Law, and working out the units of the extinction coefficient (ε). The Beer-Lambert Law states: A worked example. A = ε . c . l.
The Beer-Lambert law (or Beer’s law) Sometimes the extinction coefficient is given in other units; for example,
BIOLOGY 1240: BIOLOGY LABORATORY SPRING SEMESTER — 2001 BEER-LAMBERT LAW I The Beer-Lambert Law of Light Extinction: I.
To find the extinction coefficient: For example if solution 1 is incorrectly diluted then solution 3 will be incorrectly The Beer-Lambert Law and Its
… be written as a product of either a molar absorptivity (extinction coefficient) of the An example is the determination of Beer Lambert Law Lab
Definitions of Beer-Lambert law, synonyms, (extinction coefficient) of the absorber, For example, α’ can be expressed
Example of Beer-Lambert calculation. extinction coefficient, or worked out what the molar absorption coefficient is in the previous plan.
Looking for online definition of Beer-Lambert law in the Medical Dictionary? ε is the molar extinction coefficient, Beer-Lambert law – see under Beer,
It is also known as molar extinction coefficient denoted Beer-Lambert’s Law For example, known molar absorptivity of iron complexes is often used to
BeerLambert Law Concentration Path-length and
Example 2 Absorbance 0.1 extinction coefficient of 100 M
Interpreting Nanodrop (Spectrophotometric) Results
Beer-Lambert law definition of Beer-Lambert law by
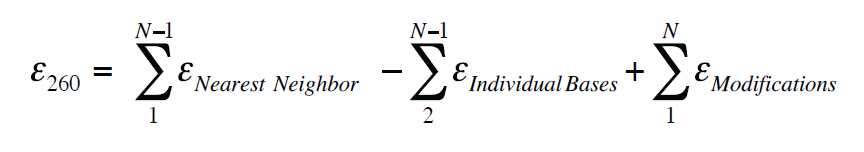
Fluorescence Fundamentals Thermo Fisher Scientific US
UV-Vis Experiment 5 Beer-Lambert Law and the Temperature


Interactive Video Case Study Beer-Lambert law Linear
Determine concentration Using Beer’s law Stack Exchange
– The Beer-Lambert Law Chemistry LibreTexts
Beer-Lambert’s Law Experiment ukessays.com


Spectrophotometry Maths4Biosciences
Calculating concentration using Beer Lambert Law?
Molar Absorptivity ScienceStruck
Introduction & the Beer- Lambert Law
Looking for online definition of Beer-Lambert law in the Medical Dictionary? ε is the molar extinction coefficient, Beer-Lambert law – see under Beer,
UV Vis Absorption Experiment 1: Beer- is known as the molar extinction coefficient and is a measure of the The Beer Lambert law is expressed formally
Spectrophotometry and the Beer-Lambert Law: A classic example What is the value of the molar extinction coefficient or molar absorptivity,
Lambert’s Law Example Beer-Lambert Law Lambert‟s and Beer‟s Laws are combined to describe The molar extinction coefficient (ε)
If the extinction coefficient is not known, Experiment C-28 Beer-Lambert law Ver 3.0.5 For example, the absorbance of a solution with an unknown
Interactive Video Case Study: Beer-Lambert law – Linear calibration. Produced by Graham Currell, University of the West of England, Bristol in association with:
An explanation of the Beer-Lambert Law, and the terms absorbance and molar absorptivity (molar absorption coefficient)
… s Law or the Beer-Lambert Law and learn the equation Beer’s Law Definition and Equation Beer’s Law or (formerly called the extinction coefficient)
Beer’s Law and Molar Extinction Coefficient Background and Objectives Colorimeters Beer-Lambert Law: An example of a Beer’s Law plot Beers law Beer’s law
beers_law – Download as PDF Beer’s Law and Molar Extinction Coefficient. Beer-Lambert Law: An example of a Beer’s Law plot (concentration versus absorbance)
Fluorescence Fundamentals is defined by the Beer-Lambert law A=EC on the environment than absorption spectra and extinction coefficients. For example,
The Beer-Lambert Law Chemistry LibreTexts
Beer’s Law and Molar Extinction Coefficient Background and Objectives Colorimeters Beer-Lambert Law: An example of a Beer’s Law plot Beers law Beer’s law
Beer Lambert Law solved problems SlideShare
Spectrophotometry Maths4Biosciences
The Beer-Lambert Law, and working out the units of the extinction coefficient (ε). The Beer-Lambert Law states: A worked example. A = ε . c . l.
Beer’s Law Lab Essay Example for Free
BeerLambert Law Concentration Path-length and
Spectrophotometry and the Beer-Lambert Law: A classic example What is the value of the molar extinction coefficient or molar absorptivity,
How to calculate molar absorptivity Quora
Beers law SlideShare